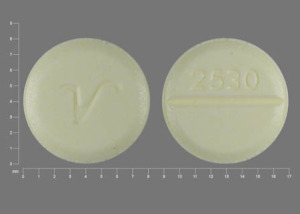
Pill with imprint V 2530 is Yellow, Round and has been identified as Clonazepam 0.5 mg.It es suministrado por Qualitest Pharmaceuticals Inc..
Clonazepam se usa en el tratamiento del trastorno de pánico; prevención de convulsiones; epilepsyand pertenece a las clases de medicamentos benzodiazepinas anticonvulsivos, benzodiazepinas.Hay evidencia positiva de riesgo fetal humano durante el embarazo.Clonazepam 0.5 mg se clasifica como una sustancia controlada de la lista 4 Bajo la Ley de sustancias controladas (CSA).,
Images for V 2530



Clonazepam
Imprint V 2530 Strength 0.5 mg Color Yellow Size 8.00 mm Shape Round Availability Prescription only Drug Class Benzodiazepine anticonvulsants, Benzodiazepines Pregnancy Category D – Positive evidence of risk CSA Schedule 4 – Some potential for abuse Labeler / Supplier Qualitest Pharmaceuticals Inc., Inactive Ingredients silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, D&C Yellow No. 10
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00603-2948 | Qualitest Pharmaceuticals |
| 67544-0697 | Prepak Systems Inc. (repackager) |
| 55289-0599 | PDRX Pharmaceuticals Inc., (repackager) |
Get help with Imprint Code FAQs.,
Consumer resources
- Clonazepam
- Clonazepam (Advanced Reading)
- Clonazepam Orally Disintegrating Tablets
- Clonazepam Tablets
Other brands: Klonopin, Klonopin Wafer
Professional resources
- Clonazepam (AHFS Monograph)
- .,.. + 3 Más
guías de tratamiento relacionadas
- abstinencia de benzodiacepinas
- ansiedad
- Trastorno Límite de la personalidad
- trastorno Bipolar
- … +15 más
Deja una respuesta